Abstract
Introduction: Outcomes for transplant-ineligible R/R DLBCL patients remain poor. Pola is an antibody-drug conjugate that delivers the microtubule inhibitor MMAE to CD79b+ cells in B-NHL. Previously, we reported results from the Ph Ib/II pola + BG arms and results of the Ph II randomized arms comparing pola + BR versus BR (Matasar et al. EHA 2017; Sehn et al. ASCO 2018). Here, we report the longer-term follow-up results for these DLBCL cohorts with additional biomarker data (ClinicalTrials.gov: NCT02257567).
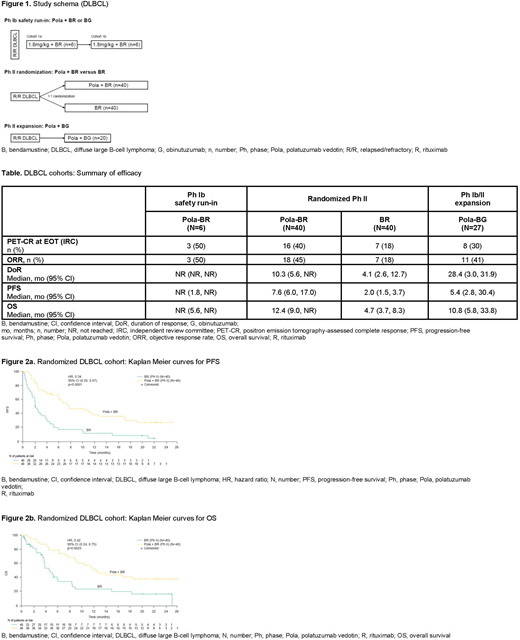
Methods: R/R DLBCL transplant ineligible patients received pola 1.8mg/kg + B (90mg/m2/day x 2 days) and R (375mg/m2) or G (1000mg). Patients were treated every 21 days for up to 6 cycles. The study design is shown in Figure 1. The randomized cohort was stratified by duration of response (DoR) to last therapy ≤12 months versus >12 months. Responses were assessed using the modified Lugano Classification (complete response [CR] required positron emission tomography (PET) negativity and negative bone marrow biopsy if positive or unknown at screening). The primary aim of Ph Ib was safety and identification of the recommended Ph II pola dose. The primary aim of the randomized Ph II component was to assess the efficacy of pola + BR vs BR at end of treatment (EOT) by an independent review committee (IRC). Other aims included evaluation of DoR, progression free survival (PFS) and overall survival (OS) with additional evaluation of efficacy by cell-of-origin (COO) and MYC/BCL2 double expression. COO was determined by Nanostring Lymph2x. MYC/BCL2 immunohistochemistry cutoffs were >40% over background and >50% with moderate to strong (2 or 3+) staining, respectively.
Results: Baseline characteristics were previously described (Matasar et al. EHA 2017; Sehn et al. ASCO 2018). As of April 30, 2018, median follow-up for the Ph Ib pola + BR, Ph II randomized pola + BR versus BR, and Ph Ib/II pola + BG arms were 37.6, 22.3, and 27.0 months, respectively.
Safety: Updated safety results are similar to those previously described with no new safety signals identified.
Efficacy: PET-CR rates at EOT by IRC and updated DoR and PFS (by investigator) and OS are shown in the Table. In the randomized Ph II portion, pola + BR showed significantly higher PET-CR rates versus BR alone (40% vs 18%; p=0.026) as assessed by IRC at EOT. Significantly longer DoR (HR, 0.44; p=0.032), PFS (HR, 0.34; p<0.0001) and OS (HR, 0.42; p=0.0023) were observed in the pola + BR arm (Figure 2). Seven (18%) patients from the P2 pola + BR cohort and 6 (23%) patients from the Ph Ib/II pola + BG cohort had responses lasting >20 months. Preliminary biomarker analyses of COO showed in the BR arm: 16 ABC and 13 GCB, and in pola + BR: 14 ABC and 14 GCB. For ABC patients, median PFS (pola + BR vs BR) was 10.5 versus 2.5 months and median OS was 13.9 versus 4.3 months, respectively. For GCB patients, median PFS (pola + BR vs BR) was 4.7 versus 1.5 months and median OS was 9.3 versus 3.2 months, respectively. Of patients tested for MYC/BCL2 overexpression, BR had 6 double expressors (DE) and 13 non-DE, and pola + BR had 9 DE and 13 non-DE. For DE, median PFS (pola + BR vs BR) was 7.0 versus 0.7 months, and median OS was 12.9 versus 3.8 months. For non-DE, median PFS (pola + BR vs BR) was 6.3 versus 2.5 months and median OS was 10.5 versus 3.8 months.
Conclusions: In a randomized setting, pola + BR showed significantly longer survival compared to BR, with median OS surpassing 12 months. In addition, results from the updated follow-up suggests that durable responses could be possible in some patients as responses of >20 months have been observed in patients treated with pola + BR/BG. Although the biomarker sample sizes were small, Pola + BR appears to provide benefit compared with BR regardless of COO or DE status. Pola + BR provides a promising treatment option for R/R DLBCL patients who are transplant ineligible.
Sehn:Celgene: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Lundbeck: Consultancy, Honoraria; Roche/Genentech: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria. Herrera:Bristol-Myers Squibb: Consultancy, Research Funding; KiTE Pharma: Consultancy, Research Funding; AstraZeneca: Research Funding; Pharmacyclics: Consultancy, Research Funding; Immune Design: Research Funding; Genentech: Consultancy, Research Funding; Gilead Sciences: Research Funding; Merck, Inc.: Consultancy, Research Funding; Seattle Genetics: Research Funding. Matasar:Seattle Genetics: Honoraria. Kamdar:Seattle Genetics: Membership on an entity's Board of Directors or advisory committees. Assouline:BMS: Honoraria, Research Funding, Speakers Bureau; Novartis: Research Funding; Roche: Honoraria, Research Funding, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Research Funding, Speakers Bureau. Hertzberg:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; MSD: Membership on an entity's Board of Directors or advisory committees. Kim:J&J: Research Funding; Celltrion: Honoraria, Research Funding; Mundipharma: Research Funding; Novartis: Research Funding; Merck: Research Funding; Celgene: Research Funding; Eisai: Honoraria, Research Funding; Takeda: Research Funding; Roche: Honoraria, Research Funding; Kyowa-Kirin: Research Funding. McMillan:Roche: Consultancy, Honoraria, Other: travel support; Takeda: Other: travel support; Amgen: Honoraria; BMS: Honoraria; Celgene: Honoraria, Other: travel support; Pfizer: Research Funding; MSD: Honoraria. Özcan:Jazz: Other: Travel support; MSD: Other: travel support, Research Funding; Jazz: Other; BMS: Honoraria; Archigen: Research Funding; Bayer: Research Funding; Celgene: Other: Travel support, Research Funding; Roche: Honoraria, Research Funding; Abbvie: Other: Travel payment; Janssen: Other: Travel Support, Research Funding; MSD: Research Funding; Takeda: Honoraria, Other: Travel payment, Research Funding; Novartis: Research Funding. Hirata:Genentech: Employment; Roche: Equity Ownership. Penuel:Genentech Inc: Employment. Cheng:Roche: Employment. Ku:Genentech: Employment. Flowers:Gilead: Consultancy; Millennium/Takeda: Research Funding; Abbvie: Research Funding; Genentech/Roche: Consultancy; Acerta: Research Funding; National Cancer Institute: Research Funding; TG Therapeutics: Research Funding; OptumRx: Consultancy; Bayer: Consultancy; Genentech/Roche: Research Funding; Karyopharm: Consultancy; Celgene: Research Funding; Spectrum: Consultancy; Pharmacyclics/ Janssen: Consultancy; Gilead: Research Funding; BeiGene: Research Funding; Abbvie: Consultancy, Research Funding; Janssen Pharmaceutical: Research Funding; Denovo Biopharma: Consultancy; Pharmacyclics: Research Funding; Burroughs Wellcome Fund: Research Funding; Eastern Cooperative Oncology Group: Research Funding; V Foundation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.